therapeutic
-
 Anti-Neuroinflammatory Natural Products from Isopod-Related Fungus Now Accessible via Chemical Synthesis
<(From left) Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim>
"Herpotrichone" is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer's disease and Parkinson's disease. This substance could only be obtained in minute quantities from fungi that are symbiotic with isopods. However, KAIST researchers have succeeded in chemically synthesizing this rare natural product, thereby presenting the possibility for the development of next-generation drugs for neurodegenerative diseases.
*Chemical Synthesis: A process of creating desired substances using chemical reactions.
KAIST (President Kwang Hyung Lee) announced on the 31st of July that a research team led by Professor Sunkyu Han of the Department of Chemistry successfully synthesized the natural anti-neuroinflammatory substances 'herpotrichones A, B, and C' for the first time.
Herpotrichone natural products are substances obtainable only in minute quantities from 'Herpotrichia sp. SF09', a symbiotic pill bug fungus, and possess a unique 6/6/6/6/3 pentacyclic framework consisting of five fused rings (four six-membered and one three-membered ring).
Interestingly, this substance exhibits excellent anti-neuroinflammatory effects that suppress brain inflammatory reactions. Recently, its mechanism of action to protect nerve cells by inhibiting ferroptosis (iron-mediated cell death) was also reported, raising expectations for its potential as a therapeutic drug for brain diseases.
Professor Han's research team devised a biosynthetically inspired strategy to chemically synthesize herpotrichoneS. The key to success was a named chemical reaction "Diels-Alder (DA) reaction". This reaction forms a six-membered ring by creating new bonds between carbon-based partners, much like two puzzle pieces interlocking to form a single ring.
<Figure 2. Key Synthetic Strategy for Hypotricon A, B, and C Based on Hydrogen Bonding>
Furthermore, the research team focused on a weak attractive phenomenon between molecules called "hydrogen bonding". By delicately designing and controlling this hydrogen bond, they were able to precisely induce the reaction to occur chemo-, regio- and stereoselectively, thereby synthesizing herpotrichone. Notably, without the pivotal hydrogen bond, only a small amount of the target natural product was formed or only undesirable byproducts were generated.
The configuration of the C2’ hydroxyl moiety was essential in directing the desired transition states leading to the target natural products.
Thanks to this induced hydrogen bonding, the reacting molecules approached the correct positions and went through an ideal transition state, allowing for the synthesis of herpotrichone C. This reaction principle was also successfully applied to herpotrichone A and B, enabling the successful synthesis of these natural products.
During the key Diels-Alder reaction conducted in the laboratory, new molecular structures not yet discovered in nature were also formed. Some of these have a high probability of being novel natural products with excellent pharmacological activity, thus doubling the significance of this research for anticipating natural products through synthesis.
Indeed, while Professor Han's research team conducted synthetic studies on herpotrichone A and B based on a 2019 paper by Chinese researchers who discovered and elucidated their structures, the research team observed the formation of undesired byproducts.
Interestingly, in 2024, the same Chinese research team that discovered herpotrichones A and bn reported the discovery of a new natural product called herpotrichone C, which turned out to be the same substance as the major byproduct previously obtained by Professor Han's team en route to herpotrichones A and B.
Professor Han stated, "This is the first total synthesis of a rare natural product with pharmacological activity related to neurodegenerative diseases and systematically presents the principle of biomimetic synthesis of complex natural products." He added, "It is expected to contribute to the development of novel natural product-based anti-neuroinflammatory therapeutics and biosynthesis research of this group of natural products."
This research outcome, with Yoojin Lee, a master's and Ph.D. integrated course student in the Department of Chemistry, as the first author, was published on July 16th in the Journal of the American Chemical Society (JACS), one of the most prestigious academic journals in the field of chemistry.
This research was supported by the National Research Foundation of Korea (NRF) Mid-career Researcher Support Program, the KAIST UP Project, the KAIST Grand Challenge 30 Project, and the KAIST Trans-Generational Collaborative Research Laboratory Project.
2025.08.04 View 390
Anti-Neuroinflammatory Natural Products from Isopod-Related Fungus Now Accessible via Chemical Synthesis
<(From left) Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim>
"Herpotrichone" is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer's disease and Parkinson's disease. This substance could only be obtained in minute quantities from fungi that are symbiotic with isopods. However, KAIST researchers have succeeded in chemically synthesizing this rare natural product, thereby presenting the possibility for the development of next-generation drugs for neurodegenerative diseases.
*Chemical Synthesis: A process of creating desired substances using chemical reactions.
KAIST (President Kwang Hyung Lee) announced on the 31st of July that a research team led by Professor Sunkyu Han of the Department of Chemistry successfully synthesized the natural anti-neuroinflammatory substances 'herpotrichones A, B, and C' for the first time.
Herpotrichone natural products are substances obtainable only in minute quantities from 'Herpotrichia sp. SF09', a symbiotic pill bug fungus, and possess a unique 6/6/6/6/3 pentacyclic framework consisting of five fused rings (four six-membered and one three-membered ring).
Interestingly, this substance exhibits excellent anti-neuroinflammatory effects that suppress brain inflammatory reactions. Recently, its mechanism of action to protect nerve cells by inhibiting ferroptosis (iron-mediated cell death) was also reported, raising expectations for its potential as a therapeutic drug for brain diseases.
Professor Han's research team devised a biosynthetically inspired strategy to chemically synthesize herpotrichoneS. The key to success was a named chemical reaction "Diels-Alder (DA) reaction". This reaction forms a six-membered ring by creating new bonds between carbon-based partners, much like two puzzle pieces interlocking to form a single ring.
<Figure 2. Key Synthetic Strategy for Hypotricon A, B, and C Based on Hydrogen Bonding>
Furthermore, the research team focused on a weak attractive phenomenon between molecules called "hydrogen bonding". By delicately designing and controlling this hydrogen bond, they were able to precisely induce the reaction to occur chemo-, regio- and stereoselectively, thereby synthesizing herpotrichone. Notably, without the pivotal hydrogen bond, only a small amount of the target natural product was formed or only undesirable byproducts were generated.
The configuration of the C2’ hydroxyl moiety was essential in directing the desired transition states leading to the target natural products.
Thanks to this induced hydrogen bonding, the reacting molecules approached the correct positions and went through an ideal transition state, allowing for the synthesis of herpotrichone C. This reaction principle was also successfully applied to herpotrichone A and B, enabling the successful synthesis of these natural products.
During the key Diels-Alder reaction conducted in the laboratory, new molecular structures not yet discovered in nature were also formed. Some of these have a high probability of being novel natural products with excellent pharmacological activity, thus doubling the significance of this research for anticipating natural products through synthesis.
Indeed, while Professor Han's research team conducted synthetic studies on herpotrichone A and B based on a 2019 paper by Chinese researchers who discovered and elucidated their structures, the research team observed the formation of undesired byproducts.
Interestingly, in 2024, the same Chinese research team that discovered herpotrichones A and bn reported the discovery of a new natural product called herpotrichone C, which turned out to be the same substance as the major byproduct previously obtained by Professor Han's team en route to herpotrichones A and B.
Professor Han stated, "This is the first total synthesis of a rare natural product with pharmacological activity related to neurodegenerative diseases and systematically presents the principle of biomimetic synthesis of complex natural products." He added, "It is expected to contribute to the development of novel natural product-based anti-neuroinflammatory therapeutics and biosynthesis research of this group of natural products."
This research outcome, with Yoojin Lee, a master's and Ph.D. integrated course student in the Department of Chemistry, as the first author, was published on July 16th in the Journal of the American Chemical Society (JACS), one of the most prestigious academic journals in the field of chemistry.
This research was supported by the National Research Foundation of Korea (NRF) Mid-career Researcher Support Program, the KAIST UP Project, the KAIST Grand Challenge 30 Project, and the KAIST Trans-Generational Collaborative Research Laboratory Project.
2025.08.04 View 390 -
 KAIST Team Develops Surface-Lighting MicroLED Patch with Significant Melanogenesis Inhibition Effect
A KAIST research team led by Ph.d candidate Jae Hee Lee and Professor Keon Jae Lee from the Department of Materials Science and Engineering has developed a surface-lighting microLED patch for UV-induced melanogenesis inhibition.
Melanin is brown or dark pigments existing in the skin, which can be abnormally synthesized by external UV or stress. Since the excessive melanin leads to skin diseases such as spots and freckles, proper treatment is required to return normal skin condition.
Recently, LED-based photo-stimulators have been released for skin care, however, their therapeutic effect is still controversial. Since conventional LED stimulators cannot conformally attach to the human skin, distance-induced side effects are caused by light loss and high heat transfer. To achieve effective phototreatment, the LED stimulator needs to be irradiated in contact with the human skin surface, enabling proper and uniform light deliver to the dermis with minimal optical loss.
In this work, the research team fabricated skin-attachable surface-lighting microLED (SµLED, 4 × 4 cm2) patch by utilizing a thousand of microLED chips and silica-embedded light diffusion layer. 100 µm-sized LED chips are vertically-interconnected for high flexibility and low heat generation, allowing its long-term operation on the human skin.
< Image 1. The overall concept of SµLED patch. a) SµLED patch operated on the human skin. b) Schematic illustration of SµLED patch structure. c) 4 × 4 cm2-sized SµLED patch. d) Schematic illustration of the advantages of SµLED patch such as efficient light delivery, low heat generation, and surface-lighting irradiation. >
The research team confirmed melanogenesis inhibition by irradiating the SµLED patch and the conventional LED (CLED) on the artificial human skin and mice dorsal skin. The SµLED-treated groups of human cells and mouse tissues showed minimal epidermal photo-toxicity and consistently effective reduction in synthesized melanin, compared to CLED-treated groups. In addition, significant suppression of proteins/catalysts expression involved in melanin synthesis such as MITF (microphthalmia-associated transcription factor), Melan-A and tyrosinase was verified.
< Image 2. The efficacy of melanogenesis inhibition on 3D human skin cells. a). Different irradiation conditions for a-MSH (major factor to stimulate melanin synthesis) treated cells. b) The ratio of pigmented area to total epidermis area. c) Relative variance of melanin level in 1 cm2-sized skin cells. A low variance means that melanin is evenly distributed, and a high variance means that the melanin is irregularly distributed. d) Optical images after in vitro experiments for 12 days. Scale bar, 1cm. e) Histological analysis of 3D skin, showing the greatest reduction in melanin after SµLED irradiation. Scale bar, 20 µm. >
< Image 3. The efficacy of melanogenesis inhibition on mouse dorsal skin. a) Optical images of mice dorsal skin after photo-treatment for 20 days. b) Histological analysis of mice dorsal skin. Less brown color means less expression of protein/catalysis involved in melanin synthesis. Scale bar, 50 µm. >
Prof. Keon Jae Lee said, “Our inorganic-based SµLED patch has outstanding characteristics in light efficiency, reliability, and durability. The SµLED patch is expected to give a great impact on the cosmetic field by reducing side effects and maximizing phototherapeutic effects.” The core technology of cosmetic SµLED has been transferred to Fronics co., Ltd, founded by Prof. Lee. Fronics is building foundry and equipment for mass production of SµLED masks for whole face cover and plans to release the products in March next year.
This paper entitled “Wearable Surface-Lighting Micro-Light-Emitting Diode Patch for Melanogenesis Inhibition” was published in the November 2022 issue of Advanced Healthcare Materials.
2022.11.22 View 14786
KAIST Team Develops Surface-Lighting MicroLED Patch with Significant Melanogenesis Inhibition Effect
A KAIST research team led by Ph.d candidate Jae Hee Lee and Professor Keon Jae Lee from the Department of Materials Science and Engineering has developed a surface-lighting microLED patch for UV-induced melanogenesis inhibition.
Melanin is brown or dark pigments existing in the skin, which can be abnormally synthesized by external UV or stress. Since the excessive melanin leads to skin diseases such as spots and freckles, proper treatment is required to return normal skin condition.
Recently, LED-based photo-stimulators have been released for skin care, however, their therapeutic effect is still controversial. Since conventional LED stimulators cannot conformally attach to the human skin, distance-induced side effects are caused by light loss and high heat transfer. To achieve effective phototreatment, the LED stimulator needs to be irradiated in contact with the human skin surface, enabling proper and uniform light deliver to the dermis with minimal optical loss.
In this work, the research team fabricated skin-attachable surface-lighting microLED (SµLED, 4 × 4 cm2) patch by utilizing a thousand of microLED chips and silica-embedded light diffusion layer. 100 µm-sized LED chips are vertically-interconnected for high flexibility and low heat generation, allowing its long-term operation on the human skin.
< Image 1. The overall concept of SµLED patch. a) SµLED patch operated on the human skin. b) Schematic illustration of SµLED patch structure. c) 4 × 4 cm2-sized SµLED patch. d) Schematic illustration of the advantages of SµLED patch such as efficient light delivery, low heat generation, and surface-lighting irradiation. >
The research team confirmed melanogenesis inhibition by irradiating the SµLED patch and the conventional LED (CLED) on the artificial human skin and mice dorsal skin. The SµLED-treated groups of human cells and mouse tissues showed minimal epidermal photo-toxicity and consistently effective reduction in synthesized melanin, compared to CLED-treated groups. In addition, significant suppression of proteins/catalysts expression involved in melanin synthesis such as MITF (microphthalmia-associated transcription factor), Melan-A and tyrosinase was verified.
< Image 2. The efficacy of melanogenesis inhibition on 3D human skin cells. a). Different irradiation conditions for a-MSH (major factor to stimulate melanin synthesis) treated cells. b) The ratio of pigmented area to total epidermis area. c) Relative variance of melanin level in 1 cm2-sized skin cells. A low variance means that melanin is evenly distributed, and a high variance means that the melanin is irregularly distributed. d) Optical images after in vitro experiments for 12 days. Scale bar, 1cm. e) Histological analysis of 3D skin, showing the greatest reduction in melanin after SµLED irradiation. Scale bar, 20 µm. >
< Image 3. The efficacy of melanogenesis inhibition on mouse dorsal skin. a) Optical images of mice dorsal skin after photo-treatment for 20 days. b) Histological analysis of mice dorsal skin. Less brown color means less expression of protein/catalysis involved in melanin synthesis. Scale bar, 50 µm. >
Prof. Keon Jae Lee said, “Our inorganic-based SµLED patch has outstanding characteristics in light efficiency, reliability, and durability. The SµLED patch is expected to give a great impact on the cosmetic field by reducing side effects and maximizing phototherapeutic effects.” The core technology of cosmetic SµLED has been transferred to Fronics co., Ltd, founded by Prof. Lee. Fronics is building foundry and equipment for mass production of SµLED masks for whole face cover and plans to release the products in March next year.
This paper entitled “Wearable Surface-Lighting Micro-Light-Emitting Diode Patch for Melanogenesis Inhibition” was published in the November 2022 issue of Advanced Healthcare Materials.
2022.11.22 View 14786 -
 Establishing a novel strategy to tackle Huntington’s disease
A platform to take on the Huntington’s disease via an innovative approach established by KAIST’s researchers through international collaboration with scientists in the Netherlands, France, and Sweden.
Through an international joint research effort involving ProQR Therapeutics of the Netherlands, Université Grenoble Alpes of France, and KTH Royal Institute of Technology of Sweden, Professor Ji-Soon Song's research team in the Department of Biological Sciences and KAIST Institute for BioCentury of KAIST, established a noble strategy to treat Huntington's disease. The new works showed that the protein converted from disease form to its disease-free form maintains its original function, providing new roadblocks to approach Huntington’s disease.
This research, titled, “A pathogenic-proteolysis resistant huntingtin isoform induced by an antisense oligonucleotide maintains huntingtin function”, co-authored by Hyeongju Kim, was published in the online edition of 'Journal of Clinical Investigation Insight' on August 9, 2022.
Huntington's disease is a dominantly inherited neurodegenerative disease and is caused by a mutation in a protein called ‘huntingtin’, which adds a distinctive feature of an expanded stretch of glutamine amino acids called polyglutamine to the protein. It is estimated that one in every 10,000 have Huntington's disease in United States. The patients would suffer a decade of regression before death, and, thus far, there is no known cure for the disease.
The cleavage near the stretched polyglutamine in mutated huntingtin is known to be the cause of the Huntington’s disease. However, as huntingtin protein is required for the development and normal function of the brain, it is critical to specifically eliminate the disease-causing protein while maintaining the ones that are still normally functioning.
The research team showed that huntingtin delta 12, the converted form of huntingtin that is resistant to developing cleavages at the ends of the protein, the known cause of the Huntington’s disease (HD), alleviated the disease’s symptoms while maintaining the functions of normal huntingtin.
Figure. Huntington's disease resistance huntingtin protein induced by antisense oligonucleotide (AON) is resistant to Caspase-6 cleavage, therefore, does not cause Huntington’s disease while maintaining normal functions of huntingtin.
The research was welcomed as it is sure to fuel innovate strategies to tackle Huntington’s disease without altering the essential function of huntingtin.
This work was supported by a Global Research Lab grant from the National Research Foundation of Korea (NRF) and by a EUREKA Eurostars 2 grant from European Union Horizon 2020.
2022.09.02 View 8932
Establishing a novel strategy to tackle Huntington’s disease
A platform to take on the Huntington’s disease via an innovative approach established by KAIST’s researchers through international collaboration with scientists in the Netherlands, France, and Sweden.
Through an international joint research effort involving ProQR Therapeutics of the Netherlands, Université Grenoble Alpes of France, and KTH Royal Institute of Technology of Sweden, Professor Ji-Soon Song's research team in the Department of Biological Sciences and KAIST Institute for BioCentury of KAIST, established a noble strategy to treat Huntington's disease. The new works showed that the protein converted from disease form to its disease-free form maintains its original function, providing new roadblocks to approach Huntington’s disease.
This research, titled, “A pathogenic-proteolysis resistant huntingtin isoform induced by an antisense oligonucleotide maintains huntingtin function”, co-authored by Hyeongju Kim, was published in the online edition of 'Journal of Clinical Investigation Insight' on August 9, 2022.
Huntington's disease is a dominantly inherited neurodegenerative disease and is caused by a mutation in a protein called ‘huntingtin’, which adds a distinctive feature of an expanded stretch of glutamine amino acids called polyglutamine to the protein. It is estimated that one in every 10,000 have Huntington's disease in United States. The patients would suffer a decade of regression before death, and, thus far, there is no known cure for the disease.
The cleavage near the stretched polyglutamine in mutated huntingtin is known to be the cause of the Huntington’s disease. However, as huntingtin protein is required for the development and normal function of the brain, it is critical to specifically eliminate the disease-causing protein while maintaining the ones that are still normally functioning.
The research team showed that huntingtin delta 12, the converted form of huntingtin that is resistant to developing cleavages at the ends of the protein, the known cause of the Huntington’s disease (HD), alleviated the disease’s symptoms while maintaining the functions of normal huntingtin.
Figure. Huntington's disease resistance huntingtin protein induced by antisense oligonucleotide (AON) is resistant to Caspase-6 cleavage, therefore, does not cause Huntington’s disease while maintaining normal functions of huntingtin.
The research was welcomed as it is sure to fuel innovate strategies to tackle Huntington’s disease without altering the essential function of huntingtin.
This work was supported by a Global Research Lab grant from the National Research Foundation of Korea (NRF) and by a EUREKA Eurostars 2 grant from European Union Horizon 2020.
2022.09.02 View 8932 -
 A New Therapeutic Drug for Alzheimer’s Disease without Inflammatory Side Effects
Although Aduhelm, a monoclonal antibody targeting amyloid beta (Aβ), recently became the first US FDA approved drug for Alzheimer’s disease (AD) based on its ability to decrease Aβ plaque burden in AD patients, its effect on cognitive improvement is still controversial. Moreover, about 40% of the patients treated with this antibody experienced serious side effects including cerebral edemas (ARIA-E) and hemorrhages (ARIA-H) that are likely related to inflammatory responses in the brain when the Aβ antibody binds Fc receptors (FCR) of immune cells such as microglia and macrophages.
These inflammatory side effects can cause neuronal cell death and synapse elimination by activated microglia, and even have the potential to exacerbate cognitive impairment in AD patients. Thus, current Aβ antibody-based immunotherapy holds the inherent risk of doing more harm than good due to their inflammatory side effects.
To overcome these problems, a team of researchers at KAIST in South Korea has developed a novel fusion protein drug, αAβ-Gas6, which efficiently eliminates Aβ via an entirely different mechanism than Aβ antibody-based immunotherapy. In a mouse model of AD, αAβ-Gas6 not only removed Aβ with higher potency, but also circumvented the neurotoxic inflammatory side effects associated with conventional antibody treatments.
Their findings were published on August 4 in Nature Medicine.
Schematic of a chimeric Gas6 fusion protein. A single chain variable fragment (scFv) of
an Amyloid β (Aβ)-targeting monoclonal antibody is fused with a truncated receptor binding
domain of Gas6, a bridging molecule for the clearance of dead cells via TAM (TYRO3, AXL,
and MERTK) receptors, which are expressed by microglia and astrocytes.
“FcR activation by Aβ targeting antibodies induces microglia-mediated Aβ phagocytosis, but it also produces inflammatory signals, inevitably damaging brain tissues,” said paper authors Chan Hyuk Kim and Won-Suk Chung, associate professors in the Department of Biological Sciences at KAIST.
“Therefore, we utilized efferocytosis, a cellular process by which dead cells are removed by phagocytes as an alternative pathway for the clearance of Aβ in the brain,” Prof. Kim and Chung said. “Efferocytosis is accompanied by anti-inflammatory responses to maintain tissue homeostasis. To exploit this process, we engineered Gas6, a soluble adaptor protein that mediates efferocytosis via TAM phagocytic receptors in such a way that its target specificity was redirected from dead cells to Aβ plaques.”
The professors and their team demonstrated that the resulting αAβ-Gas6 induced Aβ engulfment by activating not only microglial but also astrocytic phagocytosis since TAM phagocytic receptors are highly expressed by these two major phagocytes in the brain. Importantly, αAβ-Gas6 promoted the robust uptake of Aβ without showing any signs of inflammation and neurotoxicity, which contrasts sharply with the treatment using an Aβ monoclonal antibody. Moreover, they showed that αAβ-Gas6 substantially reduced excessive synapse elimination by microglia, consequently leading to better behavioral rescues in AD model mice.
“By using a mouse model of cerebral amyloid angiopathy (CAA), a cerebrovascular disorder caused by the deposition of Aβ within the walls of the brain’s blood vessels, we also showed that the intrathecal administration of Gas6 fusion protein significantly eliminated cerebrovascular amyloids, along with a reduction of microhemorrhages. These data demonstrate that aAb-Gas6 is a potent therapeutic agent in eliminating Aβ without exacerbating CAA-related microhemorrhages.”
The resulting αAβ-Gas6 clears Aβ oligomers and fibrils without causing neurotoxicity (a-b, neurons: red, and fragmented axons: yellow) and proinflammatory responses (c, TNF release), which are conversely exacerbated by the treatment of an Aβ-targeting monoclonal antibody (Aducanumab).
Professors Kim and Chung noted, “We believe our approach can be a breakthrough in treating AD without causing inflammatory side effects and synapse loss. Our approach holds promise as a novel therapeutic platform that is applicable to more than AD. By modifying the target-specificity of the fusion protein, the Gas6-fusion protein can be applied to various neurological disorders as well as autoimmune diseases affected by toxic molecules that should be removed without causing inflammatory responses.”
The number and total area of Aβ plaques (Thioflavin-T, green) were significantly reduced in αAβ-Gas6-treated AD mouse brains compared to Aducanumab-treated ones (a, b). The cognitive functions of AD model mice were significantly rescued by αAβ-Gas6 treatment, whereas Aducanumab-treated AD mice showed partial rescue in these cognitive tests (c-e).
Professors Kim and Chung founded “Illimis Therapeutics” based on this strategy of designing chimeric Gas6 fusion proteins that would remove toxic aggregates from the nervous system. Through this company, they are planning to further develop various Gas6-fusion proteins not only for Ab but also for Tau to treat AD symptoms.
This work was supported by KAIST and the Korea Health Technology R&D Project that was administered by the Korea Health Industry Development Institute (KHIDI) and the Korea Dementia Research Center (KDRC) funded by the Ministry of Health & Welfare (MOHW) and the Ministry of Science and ICT (MSIT), and KAIST.
Other contributors include Hyuncheol Jung and Se Young Lee, Sungjoon Lim, Hyeong Ryeol Choi, Yeseong Choi, Minjin Kim, Segi Kim, the Department of Biological Sciences, and the Korea Advanced Institute of Science and Technology (KAIST).
To receive more up-to-date information on this new development, follow “Illimis Therapeutics” on twitter @Illimistx.
2022.08.05 View 14891
A New Therapeutic Drug for Alzheimer’s Disease without Inflammatory Side Effects
Although Aduhelm, a monoclonal antibody targeting amyloid beta (Aβ), recently became the first US FDA approved drug for Alzheimer’s disease (AD) based on its ability to decrease Aβ plaque burden in AD patients, its effect on cognitive improvement is still controversial. Moreover, about 40% of the patients treated with this antibody experienced serious side effects including cerebral edemas (ARIA-E) and hemorrhages (ARIA-H) that are likely related to inflammatory responses in the brain when the Aβ antibody binds Fc receptors (FCR) of immune cells such as microglia and macrophages.
These inflammatory side effects can cause neuronal cell death and synapse elimination by activated microglia, and even have the potential to exacerbate cognitive impairment in AD patients. Thus, current Aβ antibody-based immunotherapy holds the inherent risk of doing more harm than good due to their inflammatory side effects.
To overcome these problems, a team of researchers at KAIST in South Korea has developed a novel fusion protein drug, αAβ-Gas6, which efficiently eliminates Aβ via an entirely different mechanism than Aβ antibody-based immunotherapy. In a mouse model of AD, αAβ-Gas6 not only removed Aβ with higher potency, but also circumvented the neurotoxic inflammatory side effects associated with conventional antibody treatments.
Their findings were published on August 4 in Nature Medicine.
Schematic of a chimeric Gas6 fusion protein. A single chain variable fragment (scFv) of
an Amyloid β (Aβ)-targeting monoclonal antibody is fused with a truncated receptor binding
domain of Gas6, a bridging molecule for the clearance of dead cells via TAM (TYRO3, AXL,
and MERTK) receptors, which are expressed by microglia and astrocytes.
“FcR activation by Aβ targeting antibodies induces microglia-mediated Aβ phagocytosis, but it also produces inflammatory signals, inevitably damaging brain tissues,” said paper authors Chan Hyuk Kim and Won-Suk Chung, associate professors in the Department of Biological Sciences at KAIST.
“Therefore, we utilized efferocytosis, a cellular process by which dead cells are removed by phagocytes as an alternative pathway for the clearance of Aβ in the brain,” Prof. Kim and Chung said. “Efferocytosis is accompanied by anti-inflammatory responses to maintain tissue homeostasis. To exploit this process, we engineered Gas6, a soluble adaptor protein that mediates efferocytosis via TAM phagocytic receptors in such a way that its target specificity was redirected from dead cells to Aβ plaques.”
The professors and their team demonstrated that the resulting αAβ-Gas6 induced Aβ engulfment by activating not only microglial but also astrocytic phagocytosis since TAM phagocytic receptors are highly expressed by these two major phagocytes in the brain. Importantly, αAβ-Gas6 promoted the robust uptake of Aβ without showing any signs of inflammation and neurotoxicity, which contrasts sharply with the treatment using an Aβ monoclonal antibody. Moreover, they showed that αAβ-Gas6 substantially reduced excessive synapse elimination by microglia, consequently leading to better behavioral rescues in AD model mice.
“By using a mouse model of cerebral amyloid angiopathy (CAA), a cerebrovascular disorder caused by the deposition of Aβ within the walls of the brain’s blood vessels, we also showed that the intrathecal administration of Gas6 fusion protein significantly eliminated cerebrovascular amyloids, along with a reduction of microhemorrhages. These data demonstrate that aAb-Gas6 is a potent therapeutic agent in eliminating Aβ without exacerbating CAA-related microhemorrhages.”
The resulting αAβ-Gas6 clears Aβ oligomers and fibrils without causing neurotoxicity (a-b, neurons: red, and fragmented axons: yellow) and proinflammatory responses (c, TNF release), which are conversely exacerbated by the treatment of an Aβ-targeting monoclonal antibody (Aducanumab).
Professors Kim and Chung noted, “We believe our approach can be a breakthrough in treating AD without causing inflammatory side effects and synapse loss. Our approach holds promise as a novel therapeutic platform that is applicable to more than AD. By modifying the target-specificity of the fusion protein, the Gas6-fusion protein can be applied to various neurological disorders as well as autoimmune diseases affected by toxic molecules that should be removed without causing inflammatory responses.”
The number and total area of Aβ plaques (Thioflavin-T, green) were significantly reduced in αAβ-Gas6-treated AD mouse brains compared to Aducanumab-treated ones (a, b). The cognitive functions of AD model mice were significantly rescued by αAβ-Gas6 treatment, whereas Aducanumab-treated AD mice showed partial rescue in these cognitive tests (c-e).
Professors Kim and Chung founded “Illimis Therapeutics” based on this strategy of designing chimeric Gas6 fusion proteins that would remove toxic aggregates from the nervous system. Through this company, they are planning to further develop various Gas6-fusion proteins not only for Ab but also for Tau to treat AD symptoms.
This work was supported by KAIST and the Korea Health Technology R&D Project that was administered by the Korea Health Industry Development Institute (KHIDI) and the Korea Dementia Research Center (KDRC) funded by the Ministry of Health & Welfare (MOHW) and the Ministry of Science and ICT (MSIT), and KAIST.
Other contributors include Hyuncheol Jung and Se Young Lee, Sungjoon Lim, Hyeong Ryeol Choi, Yeseong Choi, Minjin Kim, Segi Kim, the Department of Biological Sciences, and the Korea Advanced Institute of Science and Technology (KAIST).
To receive more up-to-date information on this new development, follow “Illimis Therapeutics” on twitter @Illimistx.
2022.08.05 View 14891 -
 Repurposed Drugs Present New Strategy for Treating COVID-19
Virtual screening of 6,218 drugs and cell-based assays identifies best therapeutic medication candidates
A joint research group from KAIST and Institut Pasteur Korea has identified repurposed drugs for COVID-19 treatment through virtual screening and cell-based assays. The research team suggested the strategy for virtual screening with greatly reduced false positives by incorporating pre-docking filtering based on shape similarity and post-docking filtering based on interaction similarity. This strategy will help develop therapeutic medications for COVID-19 and other antiviral diseases more rapidly. This study was reported at the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
Researchers screened 6,218 drugs from a collection of FDA-approved drugs or those under clinical trial and identified 38 potential repurposed drugs for COVID-19 with this strategy. Among them, seven compounds inhibited SARS-CoV-2 replication in Vero cells. Three of these drugs, emodin, omipalisib, and tipifarnib, showed anti-SARS-CoV-2 activity in human lung cells, Calu-3.
Drug repurposing is a practical strategy for developing antiviral drugs in a short period of time, especially during a global pandemic. In many instances, drug repurposing starts with the virtual screening of approved drugs. However, the actual hit rate of virtual screening is low and most of the predicted drug candidates are false positives.
The research group developed effective filtering algorithms before and after the docking simulations to improve the hit rates. In the pre-docking filtering process, compounds with similar shapes to the known active compounds for each target protein were selected and used for docking simulations. In the post-docking filtering process, the chemicals identified through their docking simulations were evaluated considering the docking energy and the similarity of the protein-ligand interactions with the known active compounds.
The experimental results showed that the virtual screening strategy reached a high hit rate of 18.4%, leading to the identification of seven potential drugs out of the 38 drugs initially selected.
“We plan to conduct further preclinical trials for optimizing drug concentrations as one of the three candidates didn’t resolve the toxicity issues in preclinical trials,” said Woo Dae Jang, one of the researchers from KAIST.
“The most important part of this research is that we developed a platform technology that can rapidly identify novel compounds for COVID-19 treatment. If we use this technology, we will be able to quickly respond to new infectious diseases as well as variants of the coronavirus,” said Distinguished Professor Sang Yup Lee.
This work was supported by the KAIST Mobile Clinic Module Project funded by the Ministry of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF). The National Culture Collection for Pathogens in Korea provided the SARS-CoV-2 (NCCP43326).
-PublicationWoo Dae Jang, Sangeun Jeon, Seungtaek Kim, and Sang Yup Lee. Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay. Proc. Natl. Acad. Sci. U.S.A. (https://doi/org/10.1073/pnas.2024302118)
-ProfileDistinguished Professor Sang Yup LeeMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2021.07.08 View 17358
Repurposed Drugs Present New Strategy for Treating COVID-19
Virtual screening of 6,218 drugs and cell-based assays identifies best therapeutic medication candidates
A joint research group from KAIST and Institut Pasteur Korea has identified repurposed drugs for COVID-19 treatment through virtual screening and cell-based assays. The research team suggested the strategy for virtual screening with greatly reduced false positives by incorporating pre-docking filtering based on shape similarity and post-docking filtering based on interaction similarity. This strategy will help develop therapeutic medications for COVID-19 and other antiviral diseases more rapidly. This study was reported at the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
Researchers screened 6,218 drugs from a collection of FDA-approved drugs or those under clinical trial and identified 38 potential repurposed drugs for COVID-19 with this strategy. Among them, seven compounds inhibited SARS-CoV-2 replication in Vero cells. Three of these drugs, emodin, omipalisib, and tipifarnib, showed anti-SARS-CoV-2 activity in human lung cells, Calu-3.
Drug repurposing is a practical strategy for developing antiviral drugs in a short period of time, especially during a global pandemic. In many instances, drug repurposing starts with the virtual screening of approved drugs. However, the actual hit rate of virtual screening is low and most of the predicted drug candidates are false positives.
The research group developed effective filtering algorithms before and after the docking simulations to improve the hit rates. In the pre-docking filtering process, compounds with similar shapes to the known active compounds for each target protein were selected and used for docking simulations. In the post-docking filtering process, the chemicals identified through their docking simulations were evaluated considering the docking energy and the similarity of the protein-ligand interactions with the known active compounds.
The experimental results showed that the virtual screening strategy reached a high hit rate of 18.4%, leading to the identification of seven potential drugs out of the 38 drugs initially selected.
“We plan to conduct further preclinical trials for optimizing drug concentrations as one of the three candidates didn’t resolve the toxicity issues in preclinical trials,” said Woo Dae Jang, one of the researchers from KAIST.
“The most important part of this research is that we developed a platform technology that can rapidly identify novel compounds for COVID-19 treatment. If we use this technology, we will be able to quickly respond to new infectious diseases as well as variants of the coronavirus,” said Distinguished Professor Sang Yup Lee.
This work was supported by the KAIST Mobile Clinic Module Project funded by the Ministry of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF). The National Culture Collection for Pathogens in Korea provided the SARS-CoV-2 (NCCP43326).
-PublicationWoo Dae Jang, Sangeun Jeon, Seungtaek Kim, and Sang Yup Lee. Drugs repurposed for COVID-19 by virtual screening of 6,218 drugs and cell-based assay. Proc. Natl. Acad. Sci. U.S.A. (https://doi/org/10.1073/pnas.2024302118)
-ProfileDistinguished Professor Sang Yup LeeMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2021.07.08 View 17358 -
 Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 19098
Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 19098 -
 Researchers Describe a Mechanism Inducing Self-Killing of Cancer Cells
(Professor Kim (left) and lead author Lee)
Researchers have described a new mechanism which induces the self-killing of cancer cells by perturbing ion homeostasis. A research team from the Department of Biochemical Engineering has developed helical polypeptide potassium ionophores that lead to the onset of programmed cell death. The ionophores increase the active oxygen concentration to stress endoplasmic reticulum to the point of cellular death.
The electrochemical gradient between extracellular and intracellular conditions plays an important role in cell growth and metabolism. When a cell’s ion homeostasis is disturbed, critical functions accelerating the activation of apoptosis are inhibited in the cell.
Although ionophores have been intensively used as an ion homeostasis disturber, the mechanisms of cell death have been unclear and the bio-applicability has been limited. In the study featured at Advanced Science, the team presented an alpha helical peptide-based anticancer agent that is capable of transporting potassium ions with water solubility. The cationic, hydrophilic, and potassium ionic groups were combined at the end of the peptide side chain to provide both ion transport and hydrophilic properties.
These peptide-based ionophores reduce the intracellular potassium concentration and at the same time increase the intracellular calcium concentration. Increased intracellular calcium concentrations produce intracellular reactive oxygen species, causing endoplasmic reticulum stress, and ultimately leading to apoptosis.
Anticancer effects were evaluated using tumor-bearing mice to confirm the therapeutic effect, even in animal models. It was found that tumor growth was strongly inhibited by endoplasmic stress-mediated apoptosis.
Lead author Dr. Dae-Yong Lee said, “A peptide-based ionophore is more effective than conventional chemotherapeutic agents because it induces apoptosis via elevated reactive oxygen species levels. Professor Yeu-Chun Kim said he expects this new mechanism to be widely used as a new chemotherapeutic strategy. This research was funded by the National Research Foundation.
2019.08.28 View 23103
Researchers Describe a Mechanism Inducing Self-Killing of Cancer Cells
(Professor Kim (left) and lead author Lee)
Researchers have described a new mechanism which induces the self-killing of cancer cells by perturbing ion homeostasis. A research team from the Department of Biochemical Engineering has developed helical polypeptide potassium ionophores that lead to the onset of programmed cell death. The ionophores increase the active oxygen concentration to stress endoplasmic reticulum to the point of cellular death.
The electrochemical gradient between extracellular and intracellular conditions plays an important role in cell growth and metabolism. When a cell’s ion homeostasis is disturbed, critical functions accelerating the activation of apoptosis are inhibited in the cell.
Although ionophores have been intensively used as an ion homeostasis disturber, the mechanisms of cell death have been unclear and the bio-applicability has been limited. In the study featured at Advanced Science, the team presented an alpha helical peptide-based anticancer agent that is capable of transporting potassium ions with water solubility. The cationic, hydrophilic, and potassium ionic groups were combined at the end of the peptide side chain to provide both ion transport and hydrophilic properties.
These peptide-based ionophores reduce the intracellular potassium concentration and at the same time increase the intracellular calcium concentration. Increased intracellular calcium concentrations produce intracellular reactive oxygen species, causing endoplasmic reticulum stress, and ultimately leading to apoptosis.
Anticancer effects were evaluated using tumor-bearing mice to confirm the therapeutic effect, even in animal models. It was found that tumor growth was strongly inhibited by endoplasmic stress-mediated apoptosis.
Lead author Dr. Dae-Yong Lee said, “A peptide-based ionophore is more effective than conventional chemotherapeutic agents because it induces apoptosis via elevated reactive oxygen species levels. Professor Yeu-Chun Kim said he expects this new mechanism to be widely used as a new chemotherapeutic strategy. This research was funded by the National Research Foundation.
2019.08.28 View 23103 -
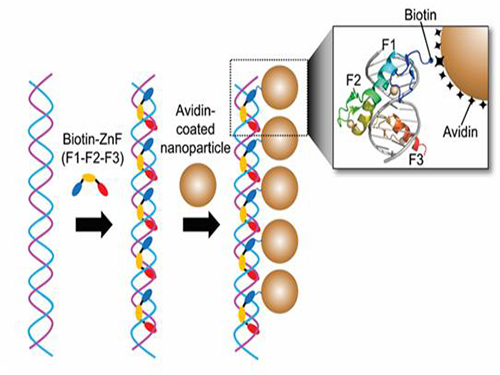 Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 15388
Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 15388 -
 Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 15104
Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 15104 -
 Six Organizations Join Forces to Induce Projected National Brain Institute to Daejeon
Six major organizations including KAIST have joined forces to help Daejeon City to win the government approval to build the envisioned Korean Brain Institute in Daedeok Research Complex.
The six organizations signed a memorandum of understanding on cooperating in establishing the government-funded institute built within the Daedeok Research Complex in the city of Daejeon, at KAIST on Jan. 14. The six organizations are KAIST, the Daejeon City Government, Korea Research Institute of Bioscience and Biotechnology, Korea Research Institute of Standard and Science, Asan Medical Center, and SK Corp., a pioneer in effective therapeutic invention for serious brain disorders.
The partnership of the six organizations is expected to bring a broad-based cooperation opportunities and create a massive synergy effect in the brain science researches and the development of new therapeutic treatment for brain disorders by combining their resources and infrastructures.
The six organizations have also built an international research network with such globally-renowned brain research institutions as RIKEN, a large natural sciences research institute in Japan, Max Plank Institute in Germany, Federal Institute of Technology, Lausanne, in Switzerland and Brain Research Institute of University of Queensland in Australia. The research network is under the support and guidance of Dennis Choi, a prominent neuroscientist who once served as the President of the Society for Neuroscience and is currently a professor in the Departments of Neurology and biology at Emory University.
The tentatively titled Korea Brain Institute is envisioned to help fight brain disorders and create Korea"s new growth engine, as well as lengthening life span, by conducting convergence researches in nero science, brain science and pharmacology. If the consortium of the six organizations wins the government approval to build the proposed institute within the Daedeok complex, the central government and the Daejeon city government are expected to pour a total of 329.7 billion won into the project by 2020.
2009.01.14 View 21927
Six Organizations Join Forces to Induce Projected National Brain Institute to Daejeon
Six major organizations including KAIST have joined forces to help Daejeon City to win the government approval to build the envisioned Korean Brain Institute in Daedeok Research Complex.
The six organizations signed a memorandum of understanding on cooperating in establishing the government-funded institute built within the Daedeok Research Complex in the city of Daejeon, at KAIST on Jan. 14. The six organizations are KAIST, the Daejeon City Government, Korea Research Institute of Bioscience and Biotechnology, Korea Research Institute of Standard and Science, Asan Medical Center, and SK Corp., a pioneer in effective therapeutic invention for serious brain disorders.
The partnership of the six organizations is expected to bring a broad-based cooperation opportunities and create a massive synergy effect in the brain science researches and the development of new therapeutic treatment for brain disorders by combining their resources and infrastructures.
The six organizations have also built an international research network with such globally-renowned brain research institutions as RIKEN, a large natural sciences research institute in Japan, Max Plank Institute in Germany, Federal Institute of Technology, Lausanne, in Switzerland and Brain Research Institute of University of Queensland in Australia. The research network is under the support and guidance of Dennis Choi, a prominent neuroscientist who once served as the President of the Society for Neuroscience and is currently a professor in the Departments of Neurology and biology at Emory University.
The tentatively titled Korea Brain Institute is envisioned to help fight brain disorders and create Korea"s new growth engine, as well as lengthening life span, by conducting convergence researches in nero science, brain science and pharmacology. If the consortium of the six organizations wins the government approval to build the proposed institute within the Daedeok complex, the central government and the Daejeon city government are expected to pour a total of 329.7 billion won into the project by 2020.
2009.01.14 View 21927