Department+of+Chemistry
-
 Distinguished Professor Sukbok Chang Donates His Prize Money
The honoree of the 2019 Korea Best Scientist and Technologist Award, Distinguished Professor Sukbok Chang donated his prize money of one hundred million KRW to the Chemistry Department Scholarship Fund and the Lyu Keun-Chul Sports Complex Management Fund during a donation ceremony last week.
Professor Chang won the award last month in recognition of his pioneering achievements and lifetime contributions to the development of carbon-hydrogen activation strategies, especially for carbon-carbon, carbon-nitrogen, and carbon-oxygen formations. Professor Chang, a world renowned chemist, has been recognized for his highly selective catalytic systems, allowing the controlled defunctionalization of bio-derived platform substrates under mild conditions and opening a new avenue for the utilization of biomass-derived platform chemicals.
“All my achievements are the results of my students’ hard work and dedication. I feel very fortunate to have such talented team members. I want to express my sincere gratitude for such a great research environment that we have worked together in so far,” said Professor Chang at the ceremony.
KAIST President Sung-Chul Shin said, “Not only will Professor Chang’s donation make a significant contribution to the Department of Chemistry, but also to the improvement of the Lyu Keun-Chul Sports Complex’s management, which directly links to the health and welfare of the KAIST community.”
Professor Chang currently holds the position of distinguished professor at KAIST and director of the Center for Catalytic Hydrocarbon Functionalizations in the Institute for Basic Science (IBS). He previously received the Kyung-Ahm Academic Award in 2013 and the Korea Toray Science Award in 2018. All these prize money also went to the school.
(END)
2019.08.26 View 8759
Distinguished Professor Sukbok Chang Donates His Prize Money
The honoree of the 2019 Korea Best Scientist and Technologist Award, Distinguished Professor Sukbok Chang donated his prize money of one hundred million KRW to the Chemistry Department Scholarship Fund and the Lyu Keun-Chul Sports Complex Management Fund during a donation ceremony last week.
Professor Chang won the award last month in recognition of his pioneering achievements and lifetime contributions to the development of carbon-hydrogen activation strategies, especially for carbon-carbon, carbon-nitrogen, and carbon-oxygen formations. Professor Chang, a world renowned chemist, has been recognized for his highly selective catalytic systems, allowing the controlled defunctionalization of bio-derived platform substrates under mild conditions and opening a new avenue for the utilization of biomass-derived platform chemicals.
“All my achievements are the results of my students’ hard work and dedication. I feel very fortunate to have such talented team members. I want to express my sincere gratitude for such a great research environment that we have worked together in so far,” said Professor Chang at the ceremony.
KAIST President Sung-Chul Shin said, “Not only will Professor Chang’s donation make a significant contribution to the Department of Chemistry, but also to the improvement of the Lyu Keun-Chul Sports Complex’s management, which directly links to the health and welfare of the KAIST community.”
Professor Chang currently holds the position of distinguished professor at KAIST and director of the Center for Catalytic Hydrocarbon Functionalizations in the Institute for Basic Science (IBS). He previously received the Kyung-Ahm Academic Award in 2013 and the Korea Toray Science Award in 2018. All these prize money also went to the school.
(END)
2019.08.26 View 8759 -
 New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 7750
New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 7750 -
 A Novel Material for Transparent and Flexible Displays
(Research team led by Professor Sang Youl Kim from the Department of Chemistry)
The next generation of flexible and transparent displays will require a high-performing and flexible polymeric material that has the optical and thermal properties of glass. The material must be transparent to visible light and have a low coefficient of thermal expansion (CTE). Unfortunately, such a polymeric material has not been available. A KAIST research team has succeeded in making a new polymeric material with an exceptionally low CTE value while retaining high transparency and excellent thermal and mechanical properties. The method developed for amorphous polymers with a controlled CTE can be applied to control the thermal expansion of organic materials as well.
Most of objects expands upon heating and shrinks by cooling, and organic polymers have a relatively large CTE compared to that of ceramics or metals. Thin, light-weight planar substrates for semiconductor devices should have a similar CTE of ceramics. Otherwise, the device can be cracked due to the stress caused by thermal expansion and contraction. Therefore, matching the CTE of the semiconductor device and the substrate is crucial for successful manufacturing of display devices. Forming a network structure by connecting polymer chains is a well-known method of reducing the CTE of amorphous polymers. However, polymers with a network structure eventually lose their flexibility and becomes brittle.
As an alternative method, Professor Sang Youl Kim from the Department of Chemistry and his team chose to adjust the distance and interaction between polymer chains. Thermal expansion and contraction of polymer films can be minimized by introducing interaction forces between the polymer chains and by arranging the direction of the force perpendicularly. The team successfully implemented this approach by appropriately designing the chemical structure of a transparent polymeric material. It is called poly (amide-imide) film, which is a transparent, flexible, and high-performing polymeric material. It is thermally stable enough to be used in the AMOLED (active-matrix organic light-emitting diode) fabrication process (stable at >400℃) with a low CTE (4ppm/℃).
The team made IGZO TFT (Indium Gallium Zinc Oxide Thin Film Transistor) devices on the newly synthesized transparent poly(amide-imide) film, and confirmed that the device could indeed operate normally even when it is folded down to a radius of 1mm.
Professor Kim said, “Our results suggest a way of controlling the thermal expansion of amorphous polymers similar to a level of glass without chemical cross-linking, which has long been regarded as a challenging problem. At the same time, we succeeded in making the polymer transparent and flexible. We expect that it can be applied to controlling the thermal expansion of various organic materials.”
This research, led by researchers Sun Dal Kim and Byungyoung Lee, was published in Science Advances on October 26. (DOI: 10.1126/sciadv.aau1956v)
2019.01.24 View 6836
A Novel Material for Transparent and Flexible Displays
(Research team led by Professor Sang Youl Kim from the Department of Chemistry)
The next generation of flexible and transparent displays will require a high-performing and flexible polymeric material that has the optical and thermal properties of glass. The material must be transparent to visible light and have a low coefficient of thermal expansion (CTE). Unfortunately, such a polymeric material has not been available. A KAIST research team has succeeded in making a new polymeric material with an exceptionally low CTE value while retaining high transparency and excellent thermal and mechanical properties. The method developed for amorphous polymers with a controlled CTE can be applied to control the thermal expansion of organic materials as well.
Most of objects expands upon heating and shrinks by cooling, and organic polymers have a relatively large CTE compared to that of ceramics or metals. Thin, light-weight planar substrates for semiconductor devices should have a similar CTE of ceramics. Otherwise, the device can be cracked due to the stress caused by thermal expansion and contraction. Therefore, matching the CTE of the semiconductor device and the substrate is crucial for successful manufacturing of display devices. Forming a network structure by connecting polymer chains is a well-known method of reducing the CTE of amorphous polymers. However, polymers with a network structure eventually lose their flexibility and becomes brittle.
As an alternative method, Professor Sang Youl Kim from the Department of Chemistry and his team chose to adjust the distance and interaction between polymer chains. Thermal expansion and contraction of polymer films can be minimized by introducing interaction forces between the polymer chains and by arranging the direction of the force perpendicularly. The team successfully implemented this approach by appropriately designing the chemical structure of a transparent polymeric material. It is called poly (amide-imide) film, which is a transparent, flexible, and high-performing polymeric material. It is thermally stable enough to be used in the AMOLED (active-matrix organic light-emitting diode) fabrication process (stable at >400℃) with a low CTE (4ppm/℃).
The team made IGZO TFT (Indium Gallium Zinc Oxide Thin Film Transistor) devices on the newly synthesized transparent poly(amide-imide) film, and confirmed that the device could indeed operate normally even when it is folded down to a radius of 1mm.
Professor Kim said, “Our results suggest a way of controlling the thermal expansion of amorphous polymers similar to a level of glass without chemical cross-linking, which has long been regarded as a challenging problem. At the same time, we succeeded in making the polymer transparent and flexible. We expect that it can be applied to controlling the thermal expansion of various organic materials.”
This research, led by researchers Sun Dal Kim and Byungyoung Lee, was published in Science Advances on October 26. (DOI: 10.1126/sciadv.aau1956v)
2019.01.24 View 6836 -
 Novel Strategies to Transform a Commercially Available Iboga Alkaloid to Post-Iboga Alkaloids
(PhD candidate HyeonggeunLim, Professor Sunkyu Han, PhD candidate Sikwang Seong)
KAIST chemists have synthesized seven different iboga and post-iboga natural products from commercially available catharanthine by mirroring nature’s biosynthetic post-modification of the iboga skeleton. They devised a novel strategy to biosynthesize the natural products via a series of selective and efficient oxidation and rearrangement reactions. This will serve as a stepping stone for developing therapeutic medications against cancer and narcotics addiction.
The research team, led by Professor Sunkyu Han, conceptualized and coined the term “Post-Iboga” alkaloids to describe the natural products that are biosynthetically derived from iboga-type alkaloids, which are composed of rearranged indole and/or isoquinuclidine backbones.
Iboga alkaloids have attracted significant attention from the scientific community due to their intriguing polycyclic structures and potential therapeutic uses against drug addictions. Nature has evolved to add architectural repertoires to this family of secondary metabolites by diversifying the iboga frameworks.
Notable examples are the FDA-approved anticancer drugs vinblastine and vincristine, both derived by the oxidative dimerization of catharanthine and vindoline subunits. Admittedly, synthetic foci toward the biosynthetic iboga-derivatives have historically been on these aforementioned dimeric natural products.
Recent natural product isolation studies on Tabernaemontana corymbosa and Ervatamia officinalis species have resulted in discoveries of various secondary metabolites that are biosynthetically derived from iboga alkaloids. These recent outbursts of iboga-derived natural product isolation reports have kindled interests toward these family of natural products.
The research team utilized (+)-catharanthine, the starting material for the industrial production of the anticancer drug Navelbine®. Well-orchestrated oxidations at the C19 position and the indole moiety of the catharanthine derivative, followed by differential rearrangements under acidic conditions, provided synthetic samples of voatinggine and tabertinggine respectively.
On the other hand, opportune oxidations at the C19 position and the alpha position of the tertiary amine moiety of the catharantine derivative, followed by a transhemiaminalization, produced the first synthetic sample of chippiine/dippinine-type natural product, dippinine B.
It is important to note that the chippiine and dippinine-type alkaloids have been targeted among synthetic chemists for over 30 years but had not succumbed to synthesis prior to this report.
Professor Han believes that their study will serve as a blueprint for further explorations of the synthesis, biosynthesis, and pharmacology of this emerging family of natural products. This study was published in Chem on November 15, 2018 (DOI: 10.1016/j.chempr.2018.10.009).
2018.11.16 View 5916
Novel Strategies to Transform a Commercially Available Iboga Alkaloid to Post-Iboga Alkaloids
(PhD candidate HyeonggeunLim, Professor Sunkyu Han, PhD candidate Sikwang Seong)
KAIST chemists have synthesized seven different iboga and post-iboga natural products from commercially available catharanthine by mirroring nature’s biosynthetic post-modification of the iboga skeleton. They devised a novel strategy to biosynthesize the natural products via a series of selective and efficient oxidation and rearrangement reactions. This will serve as a stepping stone for developing therapeutic medications against cancer and narcotics addiction.
The research team, led by Professor Sunkyu Han, conceptualized and coined the term “Post-Iboga” alkaloids to describe the natural products that are biosynthetically derived from iboga-type alkaloids, which are composed of rearranged indole and/or isoquinuclidine backbones.
Iboga alkaloids have attracted significant attention from the scientific community due to their intriguing polycyclic structures and potential therapeutic uses against drug addictions. Nature has evolved to add architectural repertoires to this family of secondary metabolites by diversifying the iboga frameworks.
Notable examples are the FDA-approved anticancer drugs vinblastine and vincristine, both derived by the oxidative dimerization of catharanthine and vindoline subunits. Admittedly, synthetic foci toward the biosynthetic iboga-derivatives have historically been on these aforementioned dimeric natural products.
Recent natural product isolation studies on Tabernaemontana corymbosa and Ervatamia officinalis species have resulted in discoveries of various secondary metabolites that are biosynthetically derived from iboga alkaloids. These recent outbursts of iboga-derived natural product isolation reports have kindled interests toward these family of natural products.
The research team utilized (+)-catharanthine, the starting material for the industrial production of the anticancer drug Navelbine®. Well-orchestrated oxidations at the C19 position and the indole moiety of the catharanthine derivative, followed by differential rearrangements under acidic conditions, provided synthetic samples of voatinggine and tabertinggine respectively.
On the other hand, opportune oxidations at the C19 position and the alpha position of the tertiary amine moiety of the catharantine derivative, followed by a transhemiaminalization, produced the first synthetic sample of chippiine/dippinine-type natural product, dippinine B.
It is important to note that the chippiine and dippinine-type alkaloids have been targeted among synthetic chemists for over 30 years but had not succumbed to synthesis prior to this report.
Professor Han believes that their study will serve as a blueprint for further explorations of the synthesis, biosynthesis, and pharmacology of this emerging family of natural products. This study was published in Chem on November 15, 2018 (DOI: 10.1016/j.chempr.2018.10.009).
2018.11.16 View 5916 -
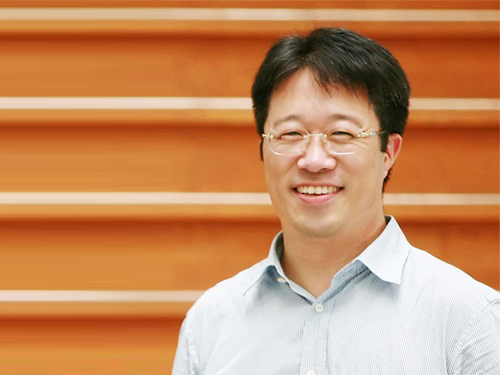 Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 5646
Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 5646 -
 Visualizing Chemical Reaction on Bimetal Surfaces
Catalysts are the result of many chemists searching to unravel the beauty of molecules and the mystery of chemical reactions. Professor Jeong Young Park from the Department of Chemistry, whose research focuses on catalytic chemical reactions, is no exception. His research team recently made breakthroughs in addressing long-standing questions for understanding reaction mechanisms on bimetal catalysts.
During the studies reported in Science Advances, following a publication in Nature Communications this month, Professor Park’s research team identified that the formation of metal–oxide interfaces is the key factor responsible for the synergistic catalytic effect in bimetal catalysts. The team confirmed this fundamental reaction mechanism through in situ imaging of reaction conditions. This is the first visualization of bimetal surfaces under reaction conditions, signifying the role of metal–oxide interfaces in heterogeneous catalysis.
Bimetallic materials have outstanding catalytic performance, which opens a new pathway for controlling electronic structures and binding energy in catalysts. Despite considerable research on various catalytic reaction efficiencies, there are yet unanswered questions on the underlying principles behind the improved performance. Even more, it was very hard to figure out what led to the efficiency because the structure, chemical composition, and oxidation state of bimetallic materials change according to reaction conditions.
Recently, some research groups suggested that oxide–metal interfacial sites formed by the surface segregation of bimetallic nanoparticles might be responsible for the increased catalytic performance. However, they failed to present any definitive evidence illustrating the physical nature or the fundamental role of the oxide–metal interfaces leading to the improved performance.
To specifically address this challenge, the research team carried out in situ observations of structural modulation on platinum–nickel bimetal catalysts under carbon monoxide oxidation conditions with ambient pressure scanning tunneling microscopy and ambient pressure X-ray photoelectron spectroscopy.
The team observed that platinum–nickel bimetal catalysts exhibited a variety of different structures depending on the gas conditions. Under ultrahigh vacuum conditions, the surface exhibited a platinum skin layer on the platinum–nickel alloyed surface, selective nickel segregation followed by the formation of nickel oxide clusters using oxygen gas, and finally the coexistence of nickel oxide clusters on the platinum skin during carbon monoxide oxidation. The research team found that the formation of interfacial platinum–nickel oxide nanostructures is responsible for a highly efficient step in the carbon monoxide oxidation reaction.
These findings illustrate that the enhancement of the catalytic activity on the bimetallic catalyst surface originates from the thermodynamically efficient reaction pathways at the metal–metal oxide interface, which demonstrates a straightforward process for the strong metal–support interaction effect. The formation of these interfacial metal–metal oxide nanostructures increases catalytic activity while providing a thermodynamically efficient reaction pathway by lowering the heat of the reactions on the surface. [J. Kim et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface, Science Advances (DOI: 10.1126/sciadv.aat3151 ]
Professor Park said that one way to monitor catalysts is to detect hot electrons associated with energy dissipation and conversion processes during surface reactions. His team led the real-time detection of hot electrons generated on bimetallic PtCo nanoparticles during exothermic hydrogen oxidation. The team successfully clarified the origin of the synergistic catalytic activity of PtCo nanoparticles with corresponding chemicurrent values.
By estimating the chemicurrent yield, the research team conclude that the catalytic properties of the bimetallic nanoparticles are strongly governed by the oxide–metal interface, which facilitates hot electron transfer. [H. Lee et al. Boosting hot electron flux and catalytic activity at metal–oxide interfaces of PtCo bimetallic nanoparticles, Nature Comm, 9, 2235 (2018)].
Professor Park explained, “We feel that the precise measurement of hot electrons on catalysts gives insight into the mechanism for heterogeneous catalysis, which can help with the smart design of highly reactive materials. The control of catalytic activity via electronic engineering of catalysts is a promising prospect that may open the door to the new field of combining catalysis with electronics, called “catalytronics.” He added that the study also establishes a strategy for improving catalytic activity for catalytic reactions in industrial chemical reactors.
Professors Park and Yousung Jung from the Department of Chemical and Biomolecular Engineering and the Graduate School of EEWS conducted this research in collaboration with Professor Bongjin Mun from the Department of Physics at GIST.
Figure 1. Evolution of surface structures of PtNi bimetal surfaces under various ambient conditions.
Figure 2. Formation of Pt-CoO interface leads to the catalytic enhancement of PtCo bimetal catalysts.
2018.07.25 View 10405
Visualizing Chemical Reaction on Bimetal Surfaces
Catalysts are the result of many chemists searching to unravel the beauty of molecules and the mystery of chemical reactions. Professor Jeong Young Park from the Department of Chemistry, whose research focuses on catalytic chemical reactions, is no exception. His research team recently made breakthroughs in addressing long-standing questions for understanding reaction mechanisms on bimetal catalysts.
During the studies reported in Science Advances, following a publication in Nature Communications this month, Professor Park’s research team identified that the formation of metal–oxide interfaces is the key factor responsible for the synergistic catalytic effect in bimetal catalysts. The team confirmed this fundamental reaction mechanism through in situ imaging of reaction conditions. This is the first visualization of bimetal surfaces under reaction conditions, signifying the role of metal–oxide interfaces in heterogeneous catalysis.
Bimetallic materials have outstanding catalytic performance, which opens a new pathway for controlling electronic structures and binding energy in catalysts. Despite considerable research on various catalytic reaction efficiencies, there are yet unanswered questions on the underlying principles behind the improved performance. Even more, it was very hard to figure out what led to the efficiency because the structure, chemical composition, and oxidation state of bimetallic materials change according to reaction conditions.
Recently, some research groups suggested that oxide–metal interfacial sites formed by the surface segregation of bimetallic nanoparticles might be responsible for the increased catalytic performance. However, they failed to present any definitive evidence illustrating the physical nature or the fundamental role of the oxide–metal interfaces leading to the improved performance.
To specifically address this challenge, the research team carried out in situ observations of structural modulation on platinum–nickel bimetal catalysts under carbon monoxide oxidation conditions with ambient pressure scanning tunneling microscopy and ambient pressure X-ray photoelectron spectroscopy.
The team observed that platinum–nickel bimetal catalysts exhibited a variety of different structures depending on the gas conditions. Under ultrahigh vacuum conditions, the surface exhibited a platinum skin layer on the platinum–nickel alloyed surface, selective nickel segregation followed by the formation of nickel oxide clusters using oxygen gas, and finally the coexistence of nickel oxide clusters on the platinum skin during carbon monoxide oxidation. The research team found that the formation of interfacial platinum–nickel oxide nanostructures is responsible for a highly efficient step in the carbon monoxide oxidation reaction.
These findings illustrate that the enhancement of the catalytic activity on the bimetallic catalyst surface originates from the thermodynamically efficient reaction pathways at the metal–metal oxide interface, which demonstrates a straightforward process for the strong metal–support interaction effect. The formation of these interfacial metal–metal oxide nanostructures increases catalytic activity while providing a thermodynamically efficient reaction pathway by lowering the heat of the reactions on the surface. [J. Kim et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface, Science Advances (DOI: 10.1126/sciadv.aat3151 ]
Professor Park said that one way to monitor catalysts is to detect hot electrons associated with energy dissipation and conversion processes during surface reactions. His team led the real-time detection of hot electrons generated on bimetallic PtCo nanoparticles during exothermic hydrogen oxidation. The team successfully clarified the origin of the synergistic catalytic activity of PtCo nanoparticles with corresponding chemicurrent values.
By estimating the chemicurrent yield, the research team conclude that the catalytic properties of the bimetallic nanoparticles are strongly governed by the oxide–metal interface, which facilitates hot electron transfer. [H. Lee et al. Boosting hot electron flux and catalytic activity at metal–oxide interfaces of PtCo bimetallic nanoparticles, Nature Comm, 9, 2235 (2018)].
Professor Park explained, “We feel that the precise measurement of hot electrons on catalysts gives insight into the mechanism for heterogeneous catalysis, which can help with the smart design of highly reactive materials. The control of catalytic activity via electronic engineering of catalysts is a promising prospect that may open the door to the new field of combining catalysis with electronics, called “catalytronics.” He added that the study also establishes a strategy for improving catalytic activity for catalytic reactions in industrial chemical reactors.
Professors Park and Yousung Jung from the Department of Chemical and Biomolecular Engineering and the Graduate School of EEWS conducted this research in collaboration with Professor Bongjin Mun from the Department of Physics at GIST.
Figure 1. Evolution of surface structures of PtNi bimetal surfaces under various ambient conditions.
Figure 2. Formation of Pt-CoO interface leads to the catalytic enhancement of PtCo bimetal catalysts.
2018.07.25 View 10405 -
 Lead-free, Efficient Perovskite for Photovoltaic Cells
(Clockwise from left: Post-doc Researcher Lamjed Debbichi, Master’s Candidate Songju Lee, Professor Min Seok Jang and Professor Hyungjun Kim)
A KAIST research team has proposed a perovskite material, Cs2Au2I6 that serves as a potential active material for highly efficient lead-free thin-film photovoltaic devices. This material is expected to lay the foundation to overcome previously known limitations of perovskite including its stability and toxicity issues.
As strong candidates for next-generation high-efficiency photovoltaic cells, perovskite photovoltaic cells have a maximum photoconversion efficiency of 22%, comparable to high-performance crystalline silicon photovoltaic cells. In addition, perovskite-based cells can be fabricated at low temperatures, thereby bringing about dramatic cost reductions.
However, it has been noted that conventional organic-inorganic hybrid perovskite materials exhibit low stability, eventually degrading their performance and making them unfit for continued use. Moreover, their inclusion of lead has undermined their environmental friendliness.
In light of this, a joint team led by Professor Hyungjun Kim from the KAIST Department of Chemistry and Professor Min Seok Jang from the School of Electrical Engineering has analyzed a previously discovered perovskite material, Cs2Au2I6, consisting of only inorganic substances and investigated its suitability for application in thin-film photovoltaic devices. Theoretical investigations suggests that this new perovskite material is not only as efficient but also more stable and environment friendly compared to the conventional perovskite materials. For this analysis, the team developed multiscale multiphysics simulation frameworks. Atomic-scale first-principle quantum calculations were carried out to study the optical properties of the proposed material, and device-scale electromagnetic simulations were conducted to suggest that the material could indeed serve as a promising photovoltaic substance at the device level.
From this point onward, the research team plans to extend the study in two directions: an empirical study to apply the perovskite material in real-world photovoltaic cells and a theoretical analysis to find the optimal and highly stable material for photovoltaic cells. The team said, “Perovskite materials are highly efficient, but in order to completely replace the conventional solar cells, their stability and toxicity issues must first be resolved.” They added that this research is expected to accelerate related studies in pursuit of high-efficiency, environment-friendly perovskite materials.
This research, led by post-doc researcher Lamjed Debbichi and master’s candidate Songju Lee, was selected as the front cover article of Advanced Materials on March 22.
Figure 1. Cover of Advanced Materials
Figure 2. Schematic of full solar cell device structure
2018.06.08 View 9617
Lead-free, Efficient Perovskite for Photovoltaic Cells
(Clockwise from left: Post-doc Researcher Lamjed Debbichi, Master’s Candidate Songju Lee, Professor Min Seok Jang and Professor Hyungjun Kim)
A KAIST research team has proposed a perovskite material, Cs2Au2I6 that serves as a potential active material for highly efficient lead-free thin-film photovoltaic devices. This material is expected to lay the foundation to overcome previously known limitations of perovskite including its stability and toxicity issues.
As strong candidates for next-generation high-efficiency photovoltaic cells, perovskite photovoltaic cells have a maximum photoconversion efficiency of 22%, comparable to high-performance crystalline silicon photovoltaic cells. In addition, perovskite-based cells can be fabricated at low temperatures, thereby bringing about dramatic cost reductions.
However, it has been noted that conventional organic-inorganic hybrid perovskite materials exhibit low stability, eventually degrading their performance and making them unfit for continued use. Moreover, their inclusion of lead has undermined their environmental friendliness.
In light of this, a joint team led by Professor Hyungjun Kim from the KAIST Department of Chemistry and Professor Min Seok Jang from the School of Electrical Engineering has analyzed a previously discovered perovskite material, Cs2Au2I6, consisting of only inorganic substances and investigated its suitability for application in thin-film photovoltaic devices. Theoretical investigations suggests that this new perovskite material is not only as efficient but also more stable and environment friendly compared to the conventional perovskite materials. For this analysis, the team developed multiscale multiphysics simulation frameworks. Atomic-scale first-principle quantum calculations were carried out to study the optical properties of the proposed material, and device-scale electromagnetic simulations were conducted to suggest that the material could indeed serve as a promising photovoltaic substance at the device level.
From this point onward, the research team plans to extend the study in two directions: an empirical study to apply the perovskite material in real-world photovoltaic cells and a theoretical analysis to find the optimal and highly stable material for photovoltaic cells. The team said, “Perovskite materials are highly efficient, but in order to completely replace the conventional solar cells, their stability and toxicity issues must first be resolved.” They added that this research is expected to accelerate related studies in pursuit of high-efficiency, environment-friendly perovskite materials.
This research, led by post-doc researcher Lamjed Debbichi and master’s candidate Songju Lee, was selected as the front cover article of Advanced Materials on March 22.
Figure 1. Cover of Advanced Materials
Figure 2. Schematic of full solar cell device structure
2018.06.08 View 9617 -
 Fast-Charging Lithium-Oxygen Batteries
(Professor Hye Ryung Byon)
KAIST researchers have paved the way for fast-charging lithium-oxygen batteries.
Professor Hye Ryung Byon from the Department of Chemistry and Professor Yousung Jung from the Graduate School of EEWS led a joint research team to develop lithium-oxygen batteries exhibiting 80% round-trip efficiency even at high charging rates, solving the problem of existing lithium-oxygen batteries which generally showed drastically lower efficiencies when the charge current rate was increased.
This study exploits the size and shape lithium peroxide, a discharge product, which is known to cause the very problems mentioned above. In doing so, the researchers have lowered the overpotential, which is the difference between the thermodynamic reversible potential and the measured potential, and simultaneously improved battery efficiency. Of particular interest is the fact that these high-performance lithium-oxygen batteries can be realized without costly catalysts.
One remarkable property of lithium-oxygen batteries is that they can accommodate three to five times the energy density of lithium-ion batteries commonly used today. Therefore, lithium-oxygen batteries would render longer driving distance to electric vehicles or drones, which operate on the continued use of electrical power.
However, their weakness lies in that, during charge, the lithium peroxide remains undecomposed at low overpotential, resulting in eventually compromising the battery’s overall performance. This is due to the poor ionic and electrical conductivity of lithium peroxide.
To tackle this issue, the researchers could form one-dimensional amorphous lithium peroxide nanostructures through the use of a mesoporous carbon electrode, CMK-3. When compared against non-mesoporous electrodes, CMK-3 showed exceptionally lower overpotential, thereby enhancing the round-trip efficiency of lithium-oxygen batteries.
The amorphous lithium peroxide produced along the electrode has a small volume and a large surface area contacting electrolyte solution, which is presumably endowed with high conductivity to speed up the charging of the lithium-oxygen batteries.
This research underpins the feasibility of overcoming the fundamental limitations of lithium-oxygen batteries even without the addition of expensive catalytic materials, but rather by the re-configuration of the size and shape of the lithium peroxide.
The findings of this research were published in Nature Communications on February 14.
Figure 1. Transmission electron microscopy (TEM) images
Figure 2. Galvanostatic rate capability
Figure 3. Density functional calculation and Bader charge analysis
2018.05.30 View 10276
Fast-Charging Lithium-Oxygen Batteries
(Professor Hye Ryung Byon)
KAIST researchers have paved the way for fast-charging lithium-oxygen batteries.
Professor Hye Ryung Byon from the Department of Chemistry and Professor Yousung Jung from the Graduate School of EEWS led a joint research team to develop lithium-oxygen batteries exhibiting 80% round-trip efficiency even at high charging rates, solving the problem of existing lithium-oxygen batteries which generally showed drastically lower efficiencies when the charge current rate was increased.
This study exploits the size and shape lithium peroxide, a discharge product, which is known to cause the very problems mentioned above. In doing so, the researchers have lowered the overpotential, which is the difference between the thermodynamic reversible potential and the measured potential, and simultaneously improved battery efficiency. Of particular interest is the fact that these high-performance lithium-oxygen batteries can be realized without costly catalysts.
One remarkable property of lithium-oxygen batteries is that they can accommodate three to five times the energy density of lithium-ion batteries commonly used today. Therefore, lithium-oxygen batteries would render longer driving distance to electric vehicles or drones, which operate on the continued use of electrical power.
However, their weakness lies in that, during charge, the lithium peroxide remains undecomposed at low overpotential, resulting in eventually compromising the battery’s overall performance. This is due to the poor ionic and electrical conductivity of lithium peroxide.
To tackle this issue, the researchers could form one-dimensional amorphous lithium peroxide nanostructures through the use of a mesoporous carbon electrode, CMK-3. When compared against non-mesoporous electrodes, CMK-3 showed exceptionally lower overpotential, thereby enhancing the round-trip efficiency of lithium-oxygen batteries.
The amorphous lithium peroxide produced along the electrode has a small volume and a large surface area contacting electrolyte solution, which is presumably endowed with high conductivity to speed up the charging of the lithium-oxygen batteries.
This research underpins the feasibility of overcoming the fundamental limitations of lithium-oxygen batteries even without the addition of expensive catalytic materials, but rather by the re-configuration of the size and shape of the lithium peroxide.
The findings of this research were published in Nature Communications on February 14.
Figure 1. Transmission electron microscopy (TEM) images
Figure 2. Galvanostatic rate capability
Figure 3. Density functional calculation and Bader charge analysis
2018.05.30 View 10276 -
 Successful Synthesis of Gamma-Lanctam Rings from Hydrocarbons
(The team of Professor Chang, far right, at the Department of Chemistry)
KAIST chemists have designed a novel strategy to synthesize ring-shaped cyclic molecules, highly sought-after by pharmaceutical and chemical industries, and known as gamma-lactams. This study describes how these five-membered rings can be prepared from inexpensive and readily available feedstock hydrocarbons, as well as from complex organic molecules, such as amino acids and steroids.
Gamma-lactams find several applications in medicinal, synthetic, and material chemistry. For example, they are included in a large number of pharmaceutically active compounds with antibiotic, anti-inflammatory, and anti-tumoral functions. This research was published in Science on March 2.
Conversion of hydrocarbons into nitrogen-containing compounds is an important area of research, where the challenge lies in breaking strong carbon-hydrogen (C−H) bonds, and converting them into carbon-nitrogen (C–N) bonds in a controlled fashion. For this reason, hydrocarbons are difficult to use as starting materials, albeit the fact that they exist in large quantities in nature.
Over the last 35 years, chemists have found ways of converting simple hydrocarbons into nitrogen-containing rings, such as indoles or pyrrolidines, but gamma-lactams proved impossible to prepare using the same approaches. Researchers hypothesized that such failure was due to alternative chemical pathways that steer the reaction away from the wanted rings: The reaction intermediate (carbonylnitrene) quickly breaks down into unsought products. Using computer models of the desired and undesired reaction pathways, the team found a strategy to completely shut down the latter in order to obtain the longed-for gamma-lactams. For the first time, these four carbons and one nitrogen cyclic molecules were obtained directly from simple feedstock chemicals.
Led by Professor Chang Sukbok at the Department of Chemistry, the team designed the winning reaction with the help of computer simulations that analyze the reaction mechanisms and calculate the energy required for the reaction to take place. According to such computer predictions, the reaction could follow three pathways, leading to the formation of either the desired gamma-lactam, an unwanted product (isocyanate), or the degradation of the catalyst caused by the substrate reacting with the catalyst backbone. Combining experimental observations and detailed computer simulations, the team designed an iridium-based catalyst, highly selective for the gamma-lactam formation. In this way, the two undesired pathways were systematically shut down, leaving the formation of the nitrogen-containing ring as the only possible outcome. Professor Chang is also in charge of the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science (IBS).
“With this work we offer a brand new solution to a long-standing challenge and demonstrate the power of what we call mechanism-based reaction development,” explains Professor Baik Mu-Hyun, a corresponding author of the study.
Beyond using cheap feedstock hydrocarbons as substrates, the team was also successful in converting amino acids, steroids, and other bio-relevant molecules into gamma-lactams, which might find a variety of applications as plant insecticide, drugs against parasitic worms, or anti-aging agents. This new synthetic technology gives much easier access to these complicated molecules and will enable the development of potential drugs in a much shorter amount of time at a lower cost.
Figure 1: Selective amidation reaction using newly designed iridium (Ir) catalysts. Abundant in nature Hydrocarbons are used as substrates to synthesize nitrogen-containing ring, called gamma-lactams.
Figure 2: Three possible reaction pathways and energy barriers predicted by computational chemistry. The scientists developed new iridium-based catalysts that are highly selective for the C–H insertion pathway which leads to the desired gamma-lactam molecules.
Figure 3: Interesting gamma-lactams derived from natural and unnatural amino acids, steroids, etc., which may be used to protect plants against insects, fight parasitic worms, or as anti-aging agents.
2018.03.02 View 8954
Successful Synthesis of Gamma-Lanctam Rings from Hydrocarbons
(The team of Professor Chang, far right, at the Department of Chemistry)
KAIST chemists have designed a novel strategy to synthesize ring-shaped cyclic molecules, highly sought-after by pharmaceutical and chemical industries, and known as gamma-lactams. This study describes how these five-membered rings can be prepared from inexpensive and readily available feedstock hydrocarbons, as well as from complex organic molecules, such as amino acids and steroids.
Gamma-lactams find several applications in medicinal, synthetic, and material chemistry. For example, they are included in a large number of pharmaceutically active compounds with antibiotic, anti-inflammatory, and anti-tumoral functions. This research was published in Science on March 2.
Conversion of hydrocarbons into nitrogen-containing compounds is an important area of research, where the challenge lies in breaking strong carbon-hydrogen (C−H) bonds, and converting them into carbon-nitrogen (C–N) bonds in a controlled fashion. For this reason, hydrocarbons are difficult to use as starting materials, albeit the fact that they exist in large quantities in nature.
Over the last 35 years, chemists have found ways of converting simple hydrocarbons into nitrogen-containing rings, such as indoles or pyrrolidines, but gamma-lactams proved impossible to prepare using the same approaches. Researchers hypothesized that such failure was due to alternative chemical pathways that steer the reaction away from the wanted rings: The reaction intermediate (carbonylnitrene) quickly breaks down into unsought products. Using computer models of the desired and undesired reaction pathways, the team found a strategy to completely shut down the latter in order to obtain the longed-for gamma-lactams. For the first time, these four carbons and one nitrogen cyclic molecules were obtained directly from simple feedstock chemicals.
Led by Professor Chang Sukbok at the Department of Chemistry, the team designed the winning reaction with the help of computer simulations that analyze the reaction mechanisms and calculate the energy required for the reaction to take place. According to such computer predictions, the reaction could follow three pathways, leading to the formation of either the desired gamma-lactam, an unwanted product (isocyanate), or the degradation of the catalyst caused by the substrate reacting with the catalyst backbone. Combining experimental observations and detailed computer simulations, the team designed an iridium-based catalyst, highly selective for the gamma-lactam formation. In this way, the two undesired pathways were systematically shut down, leaving the formation of the nitrogen-containing ring as the only possible outcome. Professor Chang is also in charge of the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science (IBS).
“With this work we offer a brand new solution to a long-standing challenge and demonstrate the power of what we call mechanism-based reaction development,” explains Professor Baik Mu-Hyun, a corresponding author of the study.
Beyond using cheap feedstock hydrocarbons as substrates, the team was also successful in converting amino acids, steroids, and other bio-relevant molecules into gamma-lactams, which might find a variety of applications as plant insecticide, drugs against parasitic worms, or anti-aging agents. This new synthetic technology gives much easier access to these complicated molecules and will enable the development of potential drugs in a much shorter amount of time at a lower cost.
Figure 1: Selective amidation reaction using newly designed iridium (Ir) catalysts. Abundant in nature Hydrocarbons are used as substrates to synthesize nitrogen-containing ring, called gamma-lactams.
Figure 2: Three possible reaction pathways and energy barriers predicted by computational chemistry. The scientists developed new iridium-based catalysts that are highly selective for the C–H insertion pathway which leads to the desired gamma-lactam molecules.
Figure 3: Interesting gamma-lactams derived from natural and unnatural amino acids, steroids, etc., which may be used to protect plants against insects, fight parasitic worms, or as anti-aging agents.
2018.03.02 View 8954 -
 New Arylation Inducing Reaction Developed
(Professor Chang(left) and Professor Baik)
KAIST researchers have identified a reaction mechanism that selectively introduces aryl groups at the desired position of a molecule at room temperature. A team, co-led by Professor Sukbok Chang and Mu-Hyun Baik of the Department of Chemistry, used an iridium catalyst for the reaction. The team also proved that the reaction proceeds by an unusual mechanism by employing computer simulations that were substantiated with targeted experimental probes.
Hydrocarbon is an omnipresent material in nature. But its low reactivity makes it difficult to process to value-added products at the room temperature. Thus, designing catalysts that can accelerate the reaction remains an important challenge in chemistry.
In particular, since most chemicals used in medicine, pharmacy, or material chemistry contain aryl groups, an effective reaction to selectively introduce the aryl group has been an area of intensive research in organic chemistry.
In order to introduce an aryl group into stable carbon-hydrogen (C-H) bond, activation of the C-H bond with a halogen atom or organic metal is required prior to the introduction of the aryl group, or C-H functionalization directly on C-H bond is needed. Direct functionalization is more effective and economical, but most reactions require harsh reaction conditions such as high temperature or excess additives. And adding the aryl fragment selectively to only one among the many possible sites in the molecule is difficult. The new catalyst developed by these KAIST researchers is highly selective.
This work is the latest example of a successful teamwork between experimental and theoretical research groups: Computer simulations revealed that traditional approaches to arylation required high energies because the intermediates produced during the reaction are too low in energy. Based on this insight, the researchers thought of changing the character of the intermediate by oxidizing it, which was predicted to be a great way of increasing the reactivity of the catalyst. Subsequent experimental work showed that this design strategy is highly effective resulting in unprecedented chemical transformations.
Professor Chang said, “We have been able to carry out location-selective arylation at room temperature, as well as identifying a new reaction pathway, different from the conventionally suggested mechanism.” He continued, “This research is significant for identifying the reaction pathway and developing a novel selective reaction method that does not require high temperature or additives based on the mechanistic understanding. This work is a triumph of rational design, rather than fortuitous discovery.” The research findings were published online in Nature Chemistry on December 11, 2017.
(Figure 1: X-ray crystal structure transmetallation intermediate)
(Figure 2: Correlation between oxidation state of intermediate and energy barrier required for reductive elimination of intermediate as calculated using density function from computational chemistry )
(Figure 3: Arylation mechanism using iridium catalyst as suggested by the research team)
2018.01.11 View 6178
New Arylation Inducing Reaction Developed
(Professor Chang(left) and Professor Baik)
KAIST researchers have identified a reaction mechanism that selectively introduces aryl groups at the desired position of a molecule at room temperature. A team, co-led by Professor Sukbok Chang and Mu-Hyun Baik of the Department of Chemistry, used an iridium catalyst for the reaction. The team also proved that the reaction proceeds by an unusual mechanism by employing computer simulations that were substantiated with targeted experimental probes.
Hydrocarbon is an omnipresent material in nature. But its low reactivity makes it difficult to process to value-added products at the room temperature. Thus, designing catalysts that can accelerate the reaction remains an important challenge in chemistry.
In particular, since most chemicals used in medicine, pharmacy, or material chemistry contain aryl groups, an effective reaction to selectively introduce the aryl group has been an area of intensive research in organic chemistry.
In order to introduce an aryl group into stable carbon-hydrogen (C-H) bond, activation of the C-H bond with a halogen atom or organic metal is required prior to the introduction of the aryl group, or C-H functionalization directly on C-H bond is needed. Direct functionalization is more effective and economical, but most reactions require harsh reaction conditions such as high temperature or excess additives. And adding the aryl fragment selectively to only one among the many possible sites in the molecule is difficult. The new catalyst developed by these KAIST researchers is highly selective.
This work is the latest example of a successful teamwork between experimental and theoretical research groups: Computer simulations revealed that traditional approaches to arylation required high energies because the intermediates produced during the reaction are too low in energy. Based on this insight, the researchers thought of changing the character of the intermediate by oxidizing it, which was predicted to be a great way of increasing the reactivity of the catalyst. Subsequent experimental work showed that this design strategy is highly effective resulting in unprecedented chemical transformations.
Professor Chang said, “We have been able to carry out location-selective arylation at room temperature, as well as identifying a new reaction pathway, different from the conventionally suggested mechanism.” He continued, “This research is significant for identifying the reaction pathway and developing a novel selective reaction method that does not require high temperature or additives based on the mechanistic understanding. This work is a triumph of rational design, rather than fortuitous discovery.” The research findings were published online in Nature Chemistry on December 11, 2017.
(Figure 1: X-ray crystal structure transmetallation intermediate)
(Figure 2: Correlation between oxidation state of intermediate and energy barrier required for reductive elimination of intermediate as calculated using density function from computational chemistry )
(Figure 3: Arylation mechanism using iridium catalyst as suggested by the research team)
2018.01.11 View 6178 -
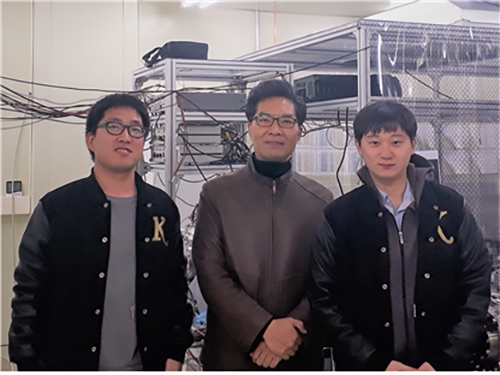 Non-Adiabatic Reaction Mechanism Identified at Conical Intersection
(Professor Kim(center) and Ph.D. candidates Kyung Chul Woo (left) and Kang Do Hyung)
Research team led by Professor Sang Kyu Kim at KAIST Department of Chemistry observed two distinct reaction pathways that occur at conical intersection where two different adiabatic potential energy surfaces cross at the same nuclear configuration.
Professor Kim previously identified the existence and molecular structure of conical intersection in 2010. In this following study, the team accurately measured reaction rates of two totally different reaction pathways activated only at conical intersection where the seminal Born-Oppenheimer approximation breaks down.
This study led by Kyung Chul Woo (1st author) and Do Hyung Kang, both Ph.D. candidates at KAIST, was published in Journal of the American Chemical Society in November 7th, 2017.
Chemical reaction induced by light occurs in excited electronic states where the reaction outcome is often destined by coupling among different electronic states mediated by nuclear motions during chemical reaction. Such a coupling is most critical and important at the conical intersection as nonadiabtic surface-hopping is most probable at situation where the Born-Oppenheimer approximation fails.
Professor Kim used spectroscopic methods in 2010 to experimentally observe conical intersection of polyatomic molecule. And yet, it was not possible to disentangle complex dynamic processes with frequency-domain study only.
The research team used pico-second time-resolution kinetic energy resolved mass spectrometry to identify two possible distinct reaction pathways in both energy and time domains.,.
The research team demonstrated that the reactive flux prepared at the conical intersection is bifurcated into adiabatic or non-adiabatic reaction pathways. These two pathways are quite distinct in terms of reaction rates, energy releases, and product branching ratios.
This is the first study to capture the moment of bifurcation dynamics at the conical intersection for complex polyatomic molecular system. The study could contribute to conceptual improvement in understanding complicated nonadiabatic dynamics in general.
Professor Kim said, “Basic science research is essential in understanding and wisely using the nature. New technological advances cannot be made without the advancement in basic science.” He continued, “I hope this study could lead to growth in many young academic talents in basic sciences.”
(Figure 1. Reaction graph starting from reaction intersection that divides into adiabatic reaction pathway (red) and non-adiabatic pathway (blue))
2017.12.19 View 6573
Non-Adiabatic Reaction Mechanism Identified at Conical Intersection
(Professor Kim(center) and Ph.D. candidates Kyung Chul Woo (left) and Kang Do Hyung)
Research team led by Professor Sang Kyu Kim at KAIST Department of Chemistry observed two distinct reaction pathways that occur at conical intersection where two different adiabatic potential energy surfaces cross at the same nuclear configuration.
Professor Kim previously identified the existence and molecular structure of conical intersection in 2010. In this following study, the team accurately measured reaction rates of two totally different reaction pathways activated only at conical intersection where the seminal Born-Oppenheimer approximation breaks down.
This study led by Kyung Chul Woo (1st author) and Do Hyung Kang, both Ph.D. candidates at KAIST, was published in Journal of the American Chemical Society in November 7th, 2017.
Chemical reaction induced by light occurs in excited electronic states where the reaction outcome is often destined by coupling among different electronic states mediated by nuclear motions during chemical reaction. Such a coupling is most critical and important at the conical intersection as nonadiabtic surface-hopping is most probable at situation where the Born-Oppenheimer approximation fails.
Professor Kim used spectroscopic methods in 2010 to experimentally observe conical intersection of polyatomic molecule. And yet, it was not possible to disentangle complex dynamic processes with frequency-domain study only.
The research team used pico-second time-resolution kinetic energy resolved mass spectrometry to identify two possible distinct reaction pathways in both energy and time domains.,.
The research team demonstrated that the reactive flux prepared at the conical intersection is bifurcated into adiabatic or non-adiabatic reaction pathways. These two pathways are quite distinct in terms of reaction rates, energy releases, and product branching ratios.
This is the first study to capture the moment of bifurcation dynamics at the conical intersection for complex polyatomic molecular system. The study could contribute to conceptual improvement in understanding complicated nonadiabatic dynamics in general.
Professor Kim said, “Basic science research is essential in understanding and wisely using the nature. New technological advances cannot be made without the advancement in basic science.” He continued, “I hope this study could lead to growth in many young academic talents in basic sciences.”
(Figure 1. Reaction graph starting from reaction intersection that divides into adiabatic reaction pathway (red) and non-adiabatic pathway (blue))
2017.12.19 View 6573 -
 New Photocatalyst Converts Carbon Dioxide to 99% Pure Fuel
(Professor Song, Ph.D. candidates Kim, and Lim (from left))
A KAIST research team led by Professor Hyunjoon Song of the Department of Chemistry developed a metal oxide nanocatalyst that converts carbon dioxide to 99% pure methane. This technology directly uses sunlight to convert carbon dioxide into methane, which is more efficient in terms of energy storage capacity, compared to the conventional way of storing the electricity produced by solar cells in batteries. The research team used cheap catalytic materials to significantly enhance the reaction efficiency and selectivity of the chemical energy storage method.
This research was conducted as a joint research project with Professor Ki Min Nam at Mokpo National University with co-first authors Dr. Kyung-Lyul Bae and Ph.D. candidates Jinmo Kim and Chan Kyu Lim. The study was published in Nature Communications on November 7.
Although there is growing interest in sunlight as an energy resource, its usage has been limited to daytime and the power output varies with the weather. If sunlight could be directly converted to chemical energy, such as fuel, the limitations of energy storage and its usage could be overcome. In particular, the usage of sunlight to convert carbon dioxide, a main cause of the greenhouse effect in our atmosphere, is of great interest since both energy and environmental issues can be addressed. However, the stability of carbon dioxide made it difficult to convert it to other molecules. Thus, there was a need for a catalyst with enhanced efficiency and selectivity.
Professor Song’s team synthesized zinc oxide nanoparticles, often used in sun cream. The nanoparticles were then bound to copper oxide as single particles, forming a colloidal form of zinc oxide-copper oxide nanoparticles. Zinc oxides produce high energy electrons using light, and this energy is used to convert carbon dioxide into methane. Further, zinc oxide can also produce electrons with light and transfer the electrons to copper oxide. Similar to the principles of photosynthesis in leaves, the electron transfer reaction could be maintained for a long time. As a consequence, although the reaction was conducted in aqueous solution, methane of 99% purity could be obtained from carbon dioxide.
Conventional heterogeneous photocatalysts were in solid powder form with irregular structures and were not dispersed in water. Professor Song’s team used a nanochemical synthesis method to control the structure of the catalyst particles to be regular and maintained over a large surface area. This led to increasing carbon dioxide conversion activity by hundreds of fold in solution compared to existing catalysts.
Professor Song said, “A long time will be needed for the commercialization of the direct conversion reaction of carbon dioxide using sunlight. However, the precise control of catalyst structures at nanoscale would enhance the efficiency of photocatalyst reactions.” He continued, “Applying this method to various phtocatalysts will maximize the catalysts performance.”
(Figure 1. Scheme for carbon dioxide conversion reaction using nano photocatalyst in aqueous solution)
(Figure 2. Structure, photocatalytic CO2 conversion, and stability of ZnO-Cu2O nanocatalyst )
2017.11.13 View 8907
New Photocatalyst Converts Carbon Dioxide to 99% Pure Fuel
(Professor Song, Ph.D. candidates Kim, and Lim (from left))
A KAIST research team led by Professor Hyunjoon Song of the Department of Chemistry developed a metal oxide nanocatalyst that converts carbon dioxide to 99% pure methane. This technology directly uses sunlight to convert carbon dioxide into methane, which is more efficient in terms of energy storage capacity, compared to the conventional way of storing the electricity produced by solar cells in batteries. The research team used cheap catalytic materials to significantly enhance the reaction efficiency and selectivity of the chemical energy storage method.
This research was conducted as a joint research project with Professor Ki Min Nam at Mokpo National University with co-first authors Dr. Kyung-Lyul Bae and Ph.D. candidates Jinmo Kim and Chan Kyu Lim. The study was published in Nature Communications on November 7.
Although there is growing interest in sunlight as an energy resource, its usage has been limited to daytime and the power output varies with the weather. If sunlight could be directly converted to chemical energy, such as fuel, the limitations of energy storage and its usage could be overcome. In particular, the usage of sunlight to convert carbon dioxide, a main cause of the greenhouse effect in our atmosphere, is of great interest since both energy and environmental issues can be addressed. However, the stability of carbon dioxide made it difficult to convert it to other molecules. Thus, there was a need for a catalyst with enhanced efficiency and selectivity.
Professor Song’s team synthesized zinc oxide nanoparticles, often used in sun cream. The nanoparticles were then bound to copper oxide as single particles, forming a colloidal form of zinc oxide-copper oxide nanoparticles. Zinc oxides produce high energy electrons using light, and this energy is used to convert carbon dioxide into methane. Further, zinc oxide can also produce electrons with light and transfer the electrons to copper oxide. Similar to the principles of photosynthesis in leaves, the electron transfer reaction could be maintained for a long time. As a consequence, although the reaction was conducted in aqueous solution, methane of 99% purity could be obtained from carbon dioxide.
Conventional heterogeneous photocatalysts were in solid powder form with irregular structures and were not dispersed in water. Professor Song’s team used a nanochemical synthesis method to control the structure of the catalyst particles to be regular and maintained over a large surface area. This led to increasing carbon dioxide conversion activity by hundreds of fold in solution compared to existing catalysts.
Professor Song said, “A long time will be needed for the commercialization of the direct conversion reaction of carbon dioxide using sunlight. However, the precise control of catalyst structures at nanoscale would enhance the efficiency of photocatalyst reactions.” He continued, “Applying this method to various phtocatalysts will maximize the catalysts performance.”
(Figure 1. Scheme for carbon dioxide conversion reaction using nano photocatalyst in aqueous solution)
(Figure 2. Structure, photocatalytic CO2 conversion, and stability of ZnO-Cu2O nanocatalyst )
2017.11.13 View 8907